 Evolving Clinical Trials with Remote Patient Monitoring (RPM)
Evolving Clinical Trials with Remote Patient Monitoring (RPM)
Evolving Clinical Trials with Remote Patient Monitoring (RPM)
In today's rapidly evolving clinical trial landscape, remote patient monitoring (RPM) is emerging as a transformative force. Leveraging digital technologies and electronic data flow (including decentralized electronic data capture and advanced analytics), digital solutions can now enable RPM of treatment intake or emergence of toxicities by healthcare providers. This is paving the way to more efficient care delivery and improved health outcomes. Here we delve into the concept of RPM in clinical trials, and its benefits for both the patients and the HCPs and in delivering better data to sponsors.
What is Remote Patient Monitoring?
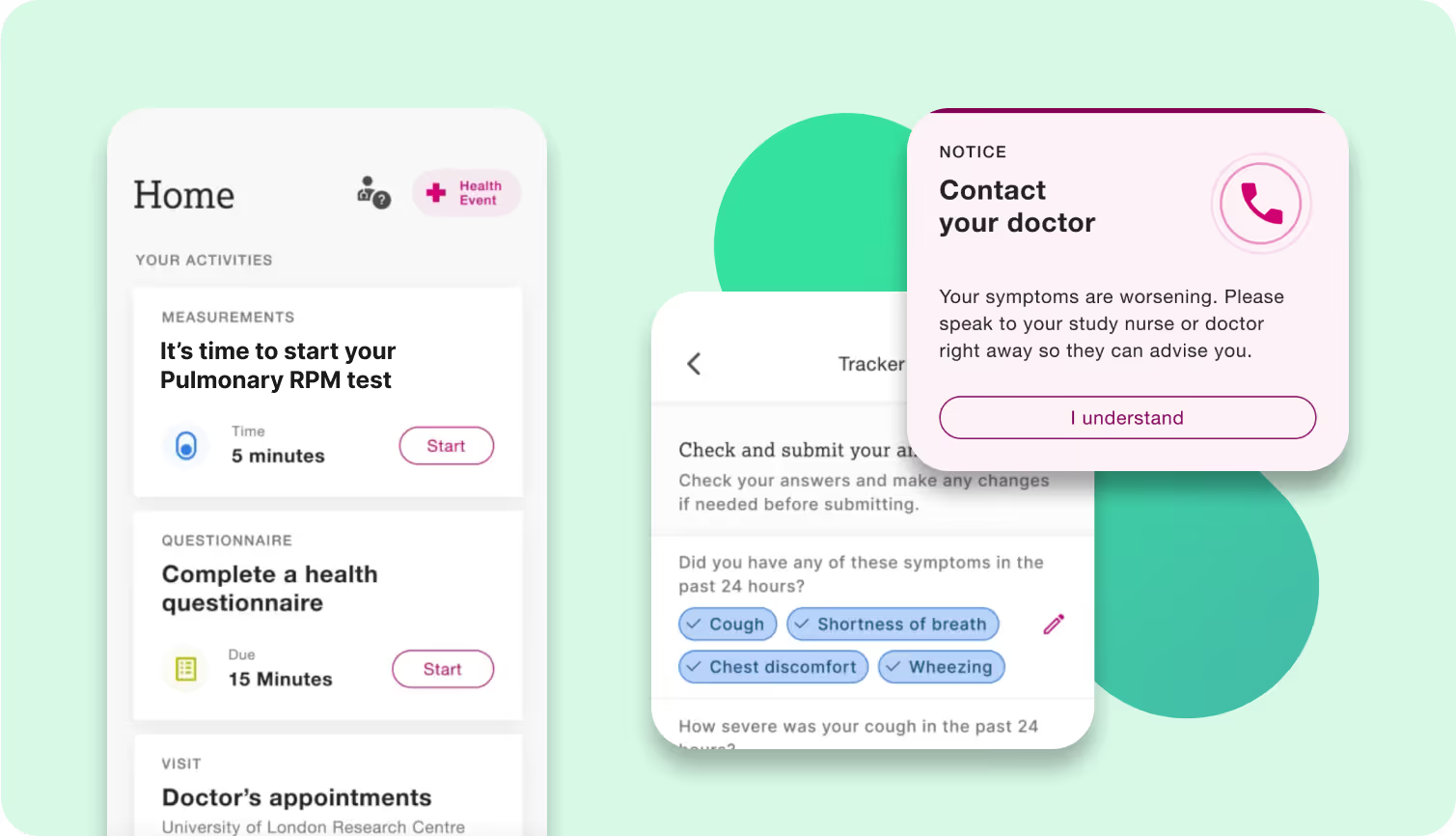
Remote Patient Monitoring (RPM) by HCPs relies on digital tools to track patients' health data outside traditional clinical setting. This approach enables real-time monitoring of outpatients' health status and treatment intake via frequent and at-home collection of physiological markers and symptoms. Associated with data-driven and medically relevant alerts for both the patients (to reach to the clinical team) or the HCP (to evaluate the patient), RPM enables early and evidence-based communication between patients and their healthcare providers. This should ultimately foster early diagnosis and management of adverse events of special interest (AESI).
RPM Data
The addition of RPM tools to a clinical trial drives an increase in data related to a specific set of parameters. This data serves multiple critical functions:
RPM as part of Risk Management Plan
Availability of reliable data throughout the course of the treatment is critical for outpatient monitoring, particularly if used as part of a strategy to mitigate morbidity, mortality and dosing impact of AESI.
Evinova RPM digital solutions advance patient-centric design and content to educate the patients on the importance of tracking regularly (in some cases daily) relevant aspects of their health status, particularly onset of symptoms; We use fit for purpose and medically relevant data collection driven by clinical consensus and measurement science. Our main focus is limiting collection burden and enabling the triaging of patients who might require clinical evaluation. Alerts, educational materials or reminders are provided as effective behavioural nudges for sustained engagement in-between clinic visits. Our healthcare provider interfaces support secured, decentralized, multi-user data access and visualization for monitoring of patients by multi-disciplinary teams.
Moving forward
Evinova digital products when implemented for the remote monitoring of patients offer pragmatic solutions for overburden site personnel to deliver care whilst mitigating risk for suboptimal dosing. Continually developing and enhancing our library of RPM modules and our product functionalities is a key part of our strategic vision of better healthcare outcomes.

Elisabeth Piault-Louis
Scientific Lead Digital Science
Transform your clinical trials today





